Following is a press release issued on November 15, 2023, reporting the results of the endTB clinical trial, which was conducted by a team of investigators, including Partners In Health (parent of Partners In Health Peru):
The results of the clinical trial presented for the first time today at The Union World Conference on Lung Health (The Union Conference 2023) revealed evidence supporting the use of four new and improved treatments for multidrug-resistant tuberculosis or rifampicin-resistant tuberculosis (MDR/XDR-TB). The team, led by Médecins Sans Frontières (MSF), Partners In Health (PIH), and Interactive Research and Development (IRD), and funded by Unitaid, formed the endTB consortium and began this Phase III randomized controlled trial in 2017.
MDR-TB is a disease caused by TB bacteria resistant to rifampicin, one of the most potent first-line antibiotics, and at least to isoniazid. Approximately half a million people become ill with MDR-TB each year, and many die from it. Although a variety of MDR-TB regimens are now used worldwide, many people are still treated with conventional treatments that are long (up to 24 months), ineffective (only 59% treatment success rate in 2018) and often cause terrible side effects, such as acute psychosis and permanent deafness. Patients under these regimens must ingest up to 14,000 pills over the entire course of treatment, and some have to endure months of painful daily injections.
New regimens, reduced treatment time
The trial found three new drug regimens that may offer similar efficacy and safety to conventional treatments while reducing treatment time by up to two-thirds. The endTB regimens represent important alternatives for short MDR-TB treatment and complement the use of another shorter, highly effective MDR-TB regimen called BPaLM, which is not suitable for certain populations. If recommended by the World Health Organization (WHO), these new patient-centered treatment regimens would allow physicians to offer shorter MDR-TB treatment regardless of age, pregnancy and comorbidities that are common among people with MDR-TB.
In addition, the trial supports the use of a fourth regimen as an alternative for people who cannot tolerate bedaquiline or linezolid; at least one of these two drugs is in every current World Health Organization-recommended regimen for MDR-TB.
The endTBtrial enrolled a diverse group of 754 patients from seven countries (Georgia, India, Kazakhstan, Lesotho, Pakistan, Peru, and South Africa). This included historically excluded populations such as adolescents and those with comorbidities such as substance use disorders, and retained participants who became pregnant during the trial. The trial evaluated five nine-month treatment regimens, and randomization was tailored according to outcome, meaning that more patients were assigned to the regimens that were producing better results.

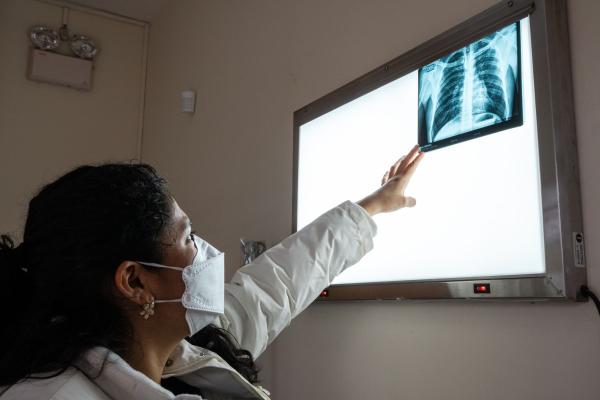
Marisol Touraine (izquierda), presidenta de Unitaid, durante su visita a Perú, donde pudo conocer, a través de Socios en Salud, a los participantes del ensayo clínico EndTB.
A breakthrough, but cost remains a barrier
“We are on the cusp of a significant advance in the fight against MDR-TB, a disease that disproportionately affects impoverished populations around the world. Our results offer hope to those in dire need and underscore the urgency of continued research and innovation, and the responsibility of private companies receiving public funds, to address diseases that too often affect the most vulnerable among us. But the cost of some drugs remains a barrier. One example is delamanid, which is still priced 12 to 40 times higher than it should be based on the independently estimated cost to produce the drug,” said Carole Mitnick, PhD (ScD), Research Director of Partners In Health for the endTB project, co-principal investigator of the study, and Professor of Global Medicine and Social Medicine at Harvard Medical School.
“For too long, MDR-TB has been a formidable threat with limited and poorly tolerated treatment options, but today we unveil evidence of multiple innovative regimens, all oral and shortened, that will enable individualized, patient-centered MDR-TB treatment. This marks a pivotal moment in the fight against a disease that has affected vulnerable populations around the world. What makes these results even more remarkable is the diversity, and resulting generalizability, of this Phase III randomized controlled trial,” said Lorenzo Guglielmetti, M.D., Médecins Sans Frontières director for the endTB project and co-principal investigator of the study.
“These results provide new hope for all those awaiting treatment for one of the most dangerous and difficult-to-treat forms of tuberculosis worldwide,” said Philippe Duneton, MD, executive director of Unitaid. “We have gold-standard research. The drugs are already available where they are needed. If recommended, this high-quality evidence could quickly translate into better treatment options suitable for all people with drug-resistant TB.”
The endTB clinical trial evaluated five experimental regimens for MDR-TB versus standard of care in two distinct analysis populations. Regimens endTB 1, 2, 3 demonstrated noninferiority to control in both primary analysis populations, establishing their success in treating MDR-TB. Regimens 1, 2, and 3 achieved favorable outcomes in 89.0%, 90.4%, and 85.2% of participants, respectively. Regimen 5 also showed a strong treatment response in 85.6% and was not inferior to 80.7% of the control in one of the primary analysis populations. While consistent results in both populations are needed to formally establish noninferiority, regimen 5 has the potential to be an alternative for patients unable to receive other recommended treatments.
Real access to these new treatment options depends on removing all barriers to receiving timely, high-quality care. The results of this trial could address a major barrier to care for many people, and the endTB consortium will continue to advocate for improved access and affordability to quality TB care.
To read more about the clinical trial results, visit endTB.org.