Socios En Salud (SES) is a leading clinical research organization in Peru. Through its Clinical Trials Unit (UNEC), it has participated in studies of high global impact such as endTB, which evaluated innovative treatments for resistant tuberculosis with results that today influence public health policies. It is also part of international networks such as SMART4TB and UNITE4TB, dedicated to the development of new clinical trials to address this disease.
This research has allowed hundreds of people to access more effective and safer treatments. But despite their benefits, clinical trials still generate doubts, fears and myths.
To resolve them, we talked with Dr. Jimena Ruiz, a UNEC physician, who answers here some of the most frequently asked questions about how these studies work and why they are a safe and ethical way to participate in science.
What is a clinical trial?
A clinical trial is a scientific investigation conducted with people -whether they are healthy or have a disease or health condition- to evaluate drugs, treatments or medical devices. Combinations of these can also be evaluated. They are carried out when there are no available treatments or when a better option than the existing one is sought. The goal is to know how safe and effective they are.
How does a clinical trial work?
In general terms, a clinical trial can be divided into three main stages:
Planning and ethical approval:
Before starting the study, the researchers draw up a protocol, which is a detailed document with the trial objectives, study design, inclusion criteria, procedures, potential risks and how those who participate will be protected. This protocol must be evaluated and approved by a research ethics committee, composed of health professionals and community members, whose role is to ensure that the study respects ethical principles and protects the rights, safety and well-being of participants.Execution and follow-up:
During this stage, individuals who meet the study criteria can participate in the trial, usually at an accredited research center. Treatments or interventions are administered under strict medical supervision, and each participant is closely monitored by a professional team, which may include physicians, nurses, laboratorians and other specialists. Follow-up is constant and may extend for months or years, depending on the type of treatment being evaluated.Analysis and reporting of results:
Once the intervention phase is over, researchers collect and analyze all the data obtained to determine whether the treatment was effective and safe. The results are shared with the scientific community through publications and presentations, and in many cases are also communicated to the individuals who participated and to the general public. This transparency is critical to the advancement of science and informed access to new therapeutic options.

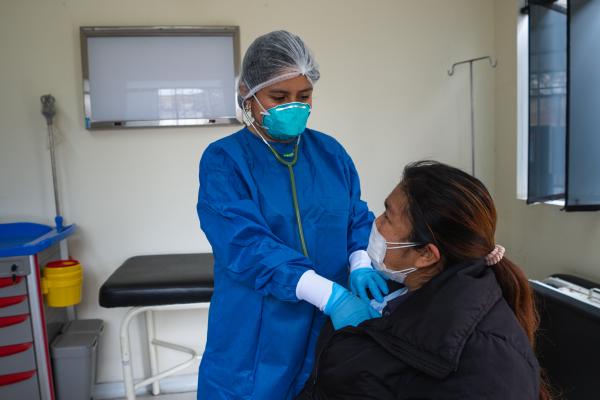
Una persona que participa en un ensayo clínico toma una decisión informada.
Foto de SES
Does participating in a clinical trial make me a “guinea pig”
No. That is an unfair and wrong comparison. Unlike a laboratory animal, a person participating in a clinical trial makes an informed decision. Before receiving any drug or intervention, a procedure called informed consent is performed. There, all the details of the study are explained, and the person can ask questions and freely decide whether he or she wants to participate or not.
Is participating in a clinical trial risky?
Every study carries risks, but in clinical trials these risks are controlled. If a new drug is administered, it is known in advance what adverse effects could occur, and the medical team is prepared to act. Moreover, if it is authorized for use, it is because the potential benefit is greater than the possible side effects. The participant is never alone: there are frequent visits, constant accompaniment and medical surveillance.
What happens if the treatment does not work or causes side effects?
If it is observed that the treatment is not working, its use is suspended. And if there are adverse effects, it can be temporarily stopped, counteracting medication given and continue to accompany the participant. People’s safety is the priority.
Can a person withdraw from a clinical trial if it has already started?
Yes. Participation is completely voluntary. A person can enter a study and, if at any time they decide they no longer want to continue, they can withdraw without explanation or penalty.
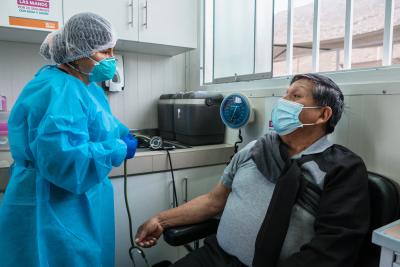
Los participantes de un ensayo clínico nunca están solos. Reciben visitas frecuentes, acompañamiento constante y vigilancia médica durante todo el proceso.
Foto de SES
Are participants informed about the results of the study?
Of course. Once the trial is completed, the results are communicated both to those who participated and to the scientific community and the general public. Knowing whether the treatment worked or not is a right of the participants.
When is placebo used instead of a real treatment?
Placebo is used only when no treatment is currently available. But if during the trial the new drug or vaccine is found to work, the participants who received the placebo can then access the real treatment.
What benefits does a person get from participating in a clinical trial?
Whoever participates in a clinical trial receives a more rigorous medical accompaniment than usual. Not only doctors and nurses are involved, but also technicians and specialists who constantly monitor their health. In addition, there is a social benefit: helping other people, in the future, to have access to better drugs, vaccines or devices. It is a concrete way to contribute to science and public health.