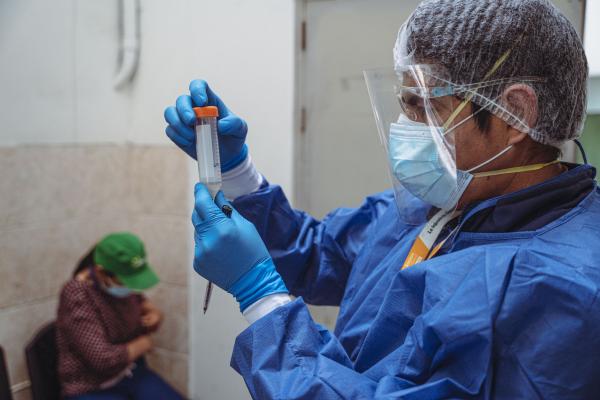
In August 2024, the U.S. National Institutes of Health (NIH) decided to fund Partners In Health (SES) research focused on the treatment of tuberculosis (TB) resistant to the drug isoniazid. A milestone in the history of our organization as it is the first project to be funded without intermediaries.
The study, which has received a Grant R01 grant, seeks to confirm that the current treatment regimen for isoniazid-resistant TB (the most common form of drug-resistant TB) is well tolerated, has few side effects and meets the WHO-recommended efficacy target (90% success rate).
The team, led by Dr. Alberto Mendoza of Socios En Salud, has as co-investigators Dr. Carole Mitnick (Harvard Medical School), Dr. Jesús Peinado (Socios En Salud), Dr. Charles Peloquin (University of Florida), SES Laboratory Director Roger Calderón, and representatives of the Peruvian National Institute of Health (INS), Zully Puyén and Miguel Grande.
Thanks to this funding, Socios En Salud will be able to advance in the evaluation of the safety, efficacy and optimization of current therapeutic regimens. Crucial support to generate new scientific evidence and improve the treatments available to combat this resistant form of TB.

El Dr. Alberto Mendoza, coordinador general del Proyecto País TB-VIH, liderará el equipo investigador que recibe financiamiento del NIH de Estados Unidos.
Foto de Diego Diaz/SES
What does SES research consist of?
Resistance of M. tuberculosis bacteria to isoniazid, the first-line drug with the most potent early bactericidal activity, is the most common form of resistant TB and reduces the efficacy of standard 6-month treatment. Based on a meta-analysis of observational reports, the learned society has recommended an alternative regimen for this type of mono-resistant TB, which includes drugs such as levofloxacin along with rifampicin, ethambutol and pyrazinamide.
However, to date, this is a weak recommendation, as it is based on evidence rated as very low quality. It is also recognized that prolonged administration of pyrazinamide carries a considerable risk of liver toxicity, and it may be necessary to increase the dose of rifampicin and levofloxacin to improve efficacy, within sufficient safety criteria, as it lacks the bactericidal action of isoniazid.
In Peru, the prevalence of isoniazid-monoresistant TB is significant. In Lima it represents 20% of cases, but half of them are still sensitive to rifampicin. Therefore, from Socios En Salud we propose a study to fill some of the gaps in the evidence supporting the safety, tolerability, effectiveness and drug dosing of this regimen.
Socios En Salud will be able to advance in the evaluation of the safety, efficacy and optimization of current therapeutic regimens.
How will the research proceed?
Through active pharmacovigilance of these patients, with biweekly phone calls and periodic medical examinations with laboratory testing, we will evaluate the safety and tolerability of the regimen. We will measure sustained effectiveness up to 12 months after admission, with periodic sputum cultures, and assess acquisition of additional resistance in individuals who fail treatment or relapse.
We will also determine the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of levofloxacin and rifampicin at week 8 in a subset of participants, additionally sampling those who fail to convert their culture to negative, to determine the minimum inhibitory concentrations of both drugs in initial isolates.
With this study, SES seeks to provide useful information on the regimen used to treat the most prevalent type of drug-resistant TB. The data will be transferable to settings with high prevalence of resistant TB. In addition, this prospective cohort design will provide high-quality evidence in a timely manner, and will strengthen the clinical research capabilities of the participating Peruvian institutions.