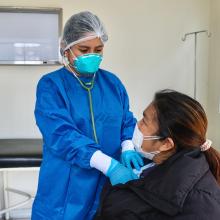
In a rapid communication, the World Health Organization (WHO) recommended three new regimens for multidrug- or rifampicin-resistant tuberculosis (MDR-MDR-TB) that were studied in the endTB clinical trial. A breakthrough in the fight against MDR-TB worldwide.
endTB is a consortium led by Partners In Health, Interactive Research and Development and Médecins Sans Frontières, and funded by Unitaid. Over the past decade, it has tested five new, all-oral, shortened treatment regimens for MDR-TB lasting only 9 months.
The short, oral endTB regimens, recommended by WHO, were studied in seven countries between 2017 and 2023, including Peru. They allow people living with MDR-TB to recover in nine months, a significant improvement over 18-month treatments that can include daily, painful injections.
“After several decades of therapeutic stagnation, and for the second time in two years, new treatments evaluated by independent stakeholders, including NGOs, have been rapidly incorporated by WHO into its recommendations to combat the scourge of multidrug-resistant TB,” said Lorenzo Guglielmetti, director of endTB at Médecins Sans Frontières and co-principal investigator of the clinical trial.
“This is a major breakthrough for the health of millions of patients affected by this particularly difficult-to-treat form of the disease. It’s important to remember that about half a million people get MDR/MDR-TB every year, and many die from it,” he adds.
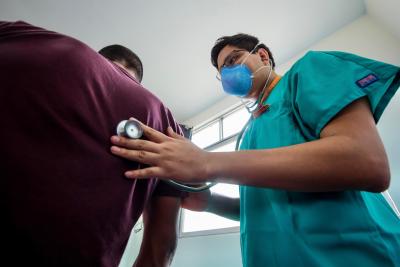
Socios En Salud colaboró con el Ministerio de Salud para llevar a cabo la implementación del nuevo régimen de tratamiento para pacientes con TB-MDR, propuesto por el brazo dos del ensayo clínico del endTB.
Other WHO-recommended treatment
The WHO also recommends the 6-month treatment strategy tested in the BEAT-TB clinical trial in South Africa. Together with the 6-month regimen known as ‘BPaLM’, recommended by WHO in 2022 following the results of the MSF-sponsored TB-PRACTECAL clinical trial, they offer a variety of long-awaited therapeutic alternatives to the long and toxic regimens used so far.
“Clinicians can now offer these advances to nearly all patients, increasing the chances of cure while reducing exposure to treatment toxicity and the spread of drug-resistant forms of TB in the community,” says Carole Mitnick, ScD, research director of Partners In Health for the endTB project, co-principal investigator of the study and professor of Global Health and Social Medicine at Harvard Medical School.
“With treatment complexity, duration and toxicity reduced, and with more options available, the prospects for eliminating the gap between need (approximately 500,000 patients per year) and the percentage treated (no more than 35% per year) improve significantly,” Mitnick highlights.
endTB in Peru
The endTB research involved 40% of all participating patients of Peruvian nationality. Its results are the first for which the WHO states that the evidence supports new recommendations for the programmatic use of novel, shortened regimens “in many population groups, including infants, adolescents, pregnant and lactating women.”
Two of the clinical trial arms of this consortium are currently being implemented in Peru. The Ministry of Health (MINSA) has initiated, between 2023 and 2024, treatments with shortened oral schemes in 112 people with MDR-TB, in health facilities in Lima and Callao.
Global To-Do
Mitnick highlights that “the four new shorter, fully oral treatments approved by WHO rely exclusively on drugs that are not under primary patents and with which physicians have significant experience.” However, two of these regimens to treat MDR/MDR-TB rely on the drug delamanid, which to date remains “extremely restricted.”
Thus, Christophe Perrin, pharmacist with MSF’s Access Campaign, calls on the Japanese corporation Otsuka, which keeps the price of this drug high through its exclusive licensee Viatris, “to stop blocking lower-priced generics from entering the market and immediately share delamanid with all companies interested in manufacturing more affordable, quality-assured generic versions of this vital TB drug.”